|
RIFE CRANE Pulsed Electric Fields |
 |
U. S. Food and Drug Administration
Center for Food Safety and Applied Nutrition
June 2, 2000
Pulsed Electric Fields
Scope of Deliverables
This section discusses current knowledge in the application of pulsed electric fields as a method of non-thermal food preservation. It includes mechanisms of inactivation, studies on microbial inactivation, critical process factors, and future research needs. Detailed descriptions of pilot and laboratory-scale equipment and their use in food preservation are also covered.
1. Introduction
1.1. Definition, Description and Applications
1.1.1 Definition
High intensity pulsed electric field (PEF) processing involves the application of pulses of high voltage (typically 20 - 80 kV/cm) to foods placed between 2 electrodes. PEF treatment is conducted at ambient, sub-ambient, or slightly above ambient temperature for less than 1 s, and energy loss due to heating of foods is minimized. For food quality attributes, PEF technology is considered superior to traditional heat treatment of foods because it avoids or greatly reduces the detrimental changes of the sensory and physical properties of foods (Quass 1997). Although some studies have concluded that PEF preserves the nutritional components of the food, effects of PEF on the chemical and nutritional aspects of foods must be better understood before it is used in food processing (Qin and others 1995b).
Some important aspects in pulsed electric field technology are the generation of high electric field intensities, the design of chambers that impart uniform treatment to foods with minimum increase in temperature, and the design of electrodes that minimize the effect of electrolysis. The large field intensities are achieved through storing a large amount of energy in a capacitor bank (a series of capacitors) from a DC power supply, which is then discharged in the form of high voltage pulses (Zhang and others 1995). Studies on energy requirements have concluded that PEF is an energy-efficient process compared to thermal pasteurization, particularly when a continuous system is used (Qin and others 1995a).
1.1.2. Description of pulsed waveforms
PEF may be applied in the form of exponentially decaying, square wave, bipolar, or oscillatory pulses. An exponential decay voltage wave is a unidirectional voltage that rises rapidly to a maximum value and decays slowly to zero. The circuit in Fig. 1 may be used to generate an exponential decay waveform. A DC power supply charges a capacitor bank connected in series with a charging resistor (Rs). When a trigger signal is applied, the charge stored in the capacitor flows though the food in the treatment chamber.
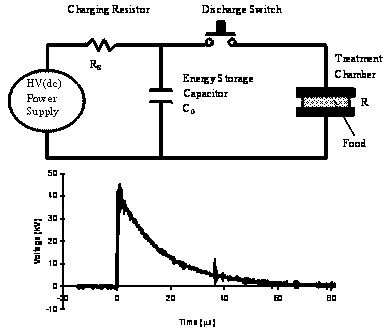 |
| Figure 1. Electrical circuit for the production of exponential decay waveforms |
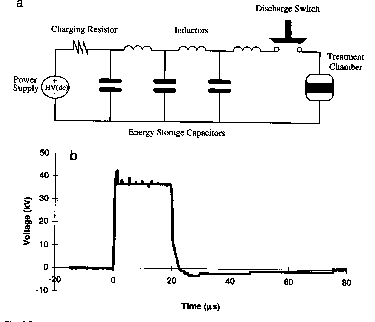 |
| Figure 2. Square pulse generator using a pulse-forming network of 3 capacitors inductor units and a voltage trace across the treatment chamber |
Square pulse waveforms are more lethal and more energy efficient than exponential decaying pulses. A square waveform can be obtained by using a pulse-forming network (PFN) consisting of an array of capacitors and inductors and solid state switching devices (Fig. 2).
The instant-charge-reversal pulses are characterized by a +ve part and -ve part (Fig. 3) with various widths and peak field strengths. An instant-charge-reversal pulse width with charge-reversal at the end of the pulse is considerably different from a standard bipolar pulse. In the latter, the polarity of the pulses is reversed alternately with relaxation time between pulses. Even with a high frequency pulser (for example, 1000 Hz), the dielectric relaxation time at zero voltage between 4 μs square wave pulses is 0.996 ms (Quass 1997). Instant-charge-reversal pulses can drastically reduce energy requirements to as low as 1.3 J/ml (EPRI 1998).
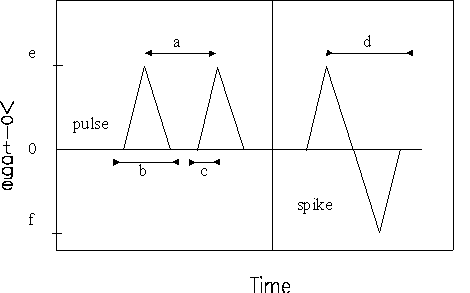 |
| Figure 3. A voltage (V) trace of an instant-charge-reversal pulse where a is pulse period (s), b is pulse width (µs), c is a pulse rise time(s) to reach e (kV), d is a spike width(s), e is a peak voltage (kV), and f is a spike voltage (kV) (Ho and others 1995). |
Oscillatory decay pulses are the least efficient, because they prevent the cell from being continuously exposed to a high intensity electric field for an extended period of time, thus preventing the cell membrane from irreversible breakdown over a large area (Jeyamkondan and others 1999).
1.1.3. Treatment chambers and equipment
Currently, there are only 2 commercial systems available (one by PurePulse Technologies, Inc. and one by Thomson-CSF). Different laboratory- and pilot-scale treatment chambers have been designed and used for PEF treatment of foods. They are classified as static (U-shaped polystyrene and glass coil static chambers) or continuous (chambers with ion conductive membrane, chambers with baffles, enhanced electric field treatment chambers, and coaxial chambers). These chambers are described in Appendix 1. A continuous flow diagram for PEF processing of foods is illustrated in Fig. 4. The test apparatus consists of 5 major components: a high-voltage power supply, an energy storage capacitor, a treatment chamber(s), a pump to conduct food though the treatment chamber(s), a cooling device, voltage, current, temperature measurement devices, and a computer to control operations.
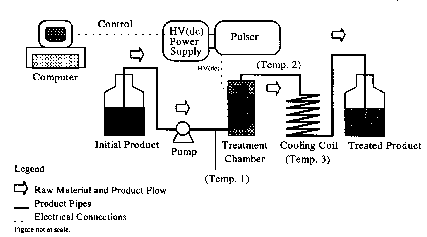 |
| Figure 4. Continuous PEF flow diagram |
1.2. Applications of PEF Technology in Food Preservation
To date, PEF has been mainly applied to preserve the quality of foods, such as to improve the shelf-life of bread, milk, orange juice, liquid eggs, and apple juice, and the fermentation properties of brewer's yeast.
1.2.1. Processing of apple juice
Simpson and others (1995) reported that apple juice from concentrate treated with PEF at 50 kV/cm, 10 pulses, pulse width of 2 µs and maximum processing temperature of 45 ° C had a shelf-life of 28 d compared to a shelf-life of 21 d of fresh-squeezed apple juice. There were no physical or chemical changes in ascorbic acid or sugars in the PEF-treated apple juice and a sensory panel found no significant differences between untreated and electric field treated juices. Vega Mercado and others (1997) reported that PEF extended the shelf-life at 22 - 25 ° C of fresh apple juice and apple juice from concentrate to more than 56 d or 32 d, respectively. There was no apparent change in its physicochemical and sensory properties.
1.2.2. Processing of orange juice
Sitzmann (1995) reported on the effectiveness of the ELSTERIL continuous process developed by the food engineers at Krupp Maachinentechnik GmbH in Hamburg, in association with the University of Hamburg, Germany. They reported the reduction of the native microbial flora of freshly squeezed orange juice by 3-log cycles with an applied electric field of 15 kV/cm without significantly affecting its quality.
Zhang and others (1997) evaluated the shelf-life of reconstituted orange juice treated with an integrated PEF pilot plant system. The PEF system consisted of a series of co-field chambers. Temperatures were maintained near ambient with cooling devices between chambers. Three waveshape pulses were used to compare the effectiveness of the processing conditions. Their results confirmed that the square wave is the most effective pulse shape. In addition, the authors reported that total aerobic counts were reduced by 3- to 4-log cycles under 32 kV/cm. When stored at 4 °C, both heat- and PEF-treated juices had a shelf-life of more than 5 mo. Vitamin C losses were lower and color was generally better preserved in PEF-treated juices compared to the heat-treated ones up to 90 d (storage temperature of 4 °C or 22 °C) or 15 d (storage temperature of 37 °C) after processing.
1.2.3. Processing of milk
Dunn and Pearlman (1987) conducted a challenge test and shelf-life study with homogenized milk inoculated with Salmonella Dublin and treated with 36.7 kV/cm and 40 pulses over a 25-min time period. Salmonella Dublin was not detected after PEF treatment or after storage at 7 - 9 ° C for 8 d. The naturally occurring milk bacterial population increased to 107 cfu/ml in the untreated milk, whereas the treated milk showed approximately 4x102 cfu/ml. Further studies by Dunn (1996) indicated less flavor degradation and no chemical or physical changes in milk quality attributes for cheesemaking. When Escherichia coli was used as the challenge bacteria, a 3-log reduction was achieved immediately after the treatment.
Fernandez-Molina and others (1999) studied the shelf-life of raw skim milk (0.2% milk fat), treated with PEF at 40 kV/cm, 30 pulses, and treatment time of 2 µs using exponential decaying pulses. The shelf-life of the milk was 2 wk stored at 4 ° C; however, treatment of raw skim milk with 80 ° C for 6 s followed by PEF treatment at 30 kV/cm, 30 pulses, and pulse width of 2 µs increased the shelf-life up to 22 d, with a total aerobic plate count of 3.6-log cfu/ml and no coliform. The processing temperature did not exceed 28 ° C during PEF treatment of the raw skim milk.
Qin and others (1995b) reported that milk (2% milk fat) subjected to 2 steps of 7 pulses each and 1 step of 6 pulses with an electric field of 40 kV/cm achieved a shelf-life of 2 wk at refrigeration temperature. There was no apparent change in its physical and chemical properties and no significant differences in sensory attributes between heat pasteurized and PEF treated milk
Calderon-Miranda (1998) studied the PEF inactivation of Listeria innocua suspended in skim milk and its subsequent sensitization to nisin. The microbial population of L. innocua was reduced by 2.5-log after PEF treatments at 30, 40 or 50 kV/cm. The same PEF intensities and subsequent exposure to 10 IU nisin/ml achieved 2-, 2.7- or 3.4-log reduction cycles of L. innocua. It appears that there may be an additional inactivation effect as a result of exposure to nisin after PEF. Reina and others (1998) studied the inactivation of Listeria monocytogenes Scott A in pasteurized whole, 2%, and skim milk with PEF. Listeria monocytogenes was reduced 1- to 3-log cycles at 25 ° C and 4-log cycles at 50 ° C, with no significant differences being found among the 3 milks. The lethal effect of PEF was a function of the field intensity and treatment time.
1.2.4. Processing of eggs
Some of the earliest studies in egg products were conducted by Dunn and Pearlman (1987) in a static parallel electrode treatment chamber with 2-cm gap using 25 exponentially decaying pulses with peak voltages of around 36 kV. Tests were carried out on liquid eggs, on heat-pasteurized liquid egg products, and on egg products with potassium sorbate and citric acid added as preservatives. Comparisons were made with regular heat-pasteurized egg products with and without the addition of food preservatives when the eggs were stored at low (4 ° C) and high (10 ° C) refrigeration temperatures. The study showed the importance of the hurdle approach in shelf-life extension. Its effectiveness was even more evident during storage at low temperatures, where egg products with a final count around 2.7 log cfu/ml stored at 10 ° C and 4 ° C maintained a low count for 4 and 10 d, respectively, versus a few hours for the heat pasteurized samples.
Other studies on liquid whole eggs (LWE) treated with PEF conducted by Qin and others (1995) and Ma and others (1997) showed that PEF treatment decreased the viscosity but increased the color (in terms of b -carotene concentration) of liquid whole eggs compared to fresh eggs. After sensory panel evaluation with a triangle test, Qin and others (1995b) found no differences between scrambled eggs prepared from fresh eggs and electric field-treated eggs; the latter were preferred over a commercial brand.
In addition to color analysis of eggs products, Ma and others (1997) evaluated the density of fresh and PEF-treated LWE (indicator of egg protein-foaming ability), as well as the strength of sponge cake baked with PEF-treated eggs. The stepwise process used by Ma and others (1997) did not cause any difference in density or whiteness between the PEF-treated and fresh LWE. The strength of the sponge cakes prepared with PEF-treated eggs was greater than the cake made with non-processed eggs. This difference in strength was attributed to the lower volume obtained after baking with PEF-treated eggs. The statistical analysis of the sensory evaluation revealed no differences between cakes prepared from PEF processed and fresh LWE.
1.2.5. Processing of green pea soup
Vega-Mercado and others (1996a) exposed pea soup to 2 steps of 16 pulses at 35 kV/cm to prevent an increase in temperature beyond 55 ° C during treatment. The shelf-life of the PEF-treated pea soup stored at refrigeration temperature exceeded 4 wk, while 22 or 32 ° C were found inappropriate to store the product. There were no apparent changes in the physical and chemical properties or sensory attributes of the pea soup directly after PEF processing or during the 4 wk of storage at refrigeration temperatures.
1.3. Current Limitations
Some of the most important current technical drawbacks or limitations of the PEF technology are:
a) The availability of commercial units, which is limited to one by PurePulse Technologies, Inc., and one by Thomson-CSF. Many pulse-power suppliers are capable of designing and constructing reliable pulsers, but except for these 2 mentioned, the complete PEF systems must be assembled independently. The systems (including treatment chambers and power supply equipments) need to be scaled up to commercial systems.
b) The presence of bubbles, which may lead to non-uniform treatment as well as operational and safety problems. When the applied electric field exceeds the dielectric strength of the gas bubbles, partial discharges take place inside the bubbles that can volatize the liquid and therefore increase the volume of the bubbles. The bubbles may become big enough to bridge the gap between the 2 electrodes and may produce a spark. Therefore, air bubbles in the food must be removed, particularly with batch systems. Vacuum degassing or pressurizing the treatment media during processing, using positive back pressure, can minimize the presence of gas. In general, however, the PEF method is not suitable for most of the solid food products containing air bubbles when placed in the treatment chamber.
c) Limited application, which is restricted to food products that can withstand high electric fields. The dielectric property of a food is closely related to its physical structure and chemical composition. Homogeneous liquids with low electrical conductivity provide ideal conditions for continuous treatment with the PEF method. Food products without the addition of salt have conductivity in the range of 0.1 to 0.5 S/m. Products with high electrical conductivity reduce the resistance of the chamber and consequently require more energy to achieve a specific electrical field. Therefore, when processing high salt products, the salt should be added after processing.
d) The particle size of the liquid food in both static and flow treatment modes. The maximum particle size in the liquid must be smaller than the gap of the treatment region in the chamber in order to maintain a proper processing operation.
e) The lack of methods to accurately measure treatment delivery. The number and diversity in equipment, limits the validity of conclusions that can be drawn about the effectiveness of particular process conditions. A method to measure treatment delivery would prevent inconsistent results due to variations in PEF systems. Such a method is not available yet.
1.4. Summary of Mechanisms of Microbial Inactivation
The application of electrical fields to biological cells in a medium (for example, water) causes buildup of electrical charges at the cell membrane (Schoenbach and others 1997). Membrane disruption occurs when the induced membrane potential exceeds a critical value of 1 V in many cellular systems, which, for example, corresponds to an external electric field of about 10 kV/cm for E. coli (Castro and others 1993). Several theories have been proposed to explain microbial inactivation by PEF. Among them, the most studied are electrical breakdown and electroporation or disruption of cell membranes (Zimermmann and Benz 1980; Zimermmann 1986; Castro and others 1993; Sale and Hamilton 1967; Vega-Mercado and others 1996a; 1996b). These theories will be explained in greater detail in Section 3.
1.5. Summary of Microbial Inactivation Kinetics
The development of mathematical models to express the inactivation kinetics of PEF is an area of research that needs extensive further work. Nevertheless, some models have been proposed and need further evaluation (see Section 3.2).
1.6. Summary of Critical Process Factors
Three types of factors that affect the microbial inactivation with PEF have been identified: factors depending on (1) the process (electric field intensity, pulse width, treatment time and temperature, and pulse waveshapes), (2) microbial entity (type, concentration, and growth stage of microorganism), and (3) treatment media (pH, antimicrobials, and ionic compounds, conductivity, and medium ionic strength).
2. Critical Process Factors and How they Impact Microbial Inactivation
2.1. Analysis of Critical Factors
2.1.1. Process factors
a) Electric field intensity. Electric field intensity is one of the main factors that influences microbial inactivation (Hüshelguer and Niemann 1980; Dunne and others 1996). The microbial inactivation increases with an increase in the electric field intensity, above the critical transmembrane potential (Qin and others 1998). This is consistent with the electroporation theory, in which the induced potential difference across the cell membrane is proportional to the applied electric field (Section 3.1.2.). Some empirical mathematical models (that is, Tables 4 and 5) have been proposed to describe the relationship between the electric field intensity and microbial inactivation. The critical electric field Ec (electric field intensity below which inactivation does not occur) increases with the transmembrane potential of the cell. Transmembrane potentials, and consequently Ec, are larger for larger cells (Jeyamkondan and others 1999). Pulse width also influences the critical electric field; for instance, with pulse widths greater than 50 µs, Ec is 4.9 kV/cm. With pulse widths less than 2 µs, Ec is 40 kV/cm (Schoenbach and others 1997).
The model of Peleg (Table 5) was used to relate the electric field intensity and applied number of pulses required to inactivate 50% of the cells (Peleg 1995).
b) Treatment time. Treatment time is defined as the product of the number pulses and the pulse duration. An increase in any of these variables increases microbial inactivation (Sale and Hamilton 1967). As noted above, pulse width influences microbial reduction by affecting Ec. Longer widths decrease Ec, which results in higher inactivation; however, an increase in pulse duration may also result in an undesirable food temperature increase. Optimum processing conditions should therefore be established to obtain the highest inactivation rate with the lowest heating effect. Hülsheger and others (1981) proposed an inactivation kinetic model that relates microbial survival fraction (S) with PEF treatment time (t). The inactivation of microorganisms increases with an increase in treatment time (Table 4; Hülsheger and others 1983). In certain cases, though, the number of pulses increasing inactivation reaches saturation. Such is the case of Saccharomyces cerevisiae inactivation by PEF that reaches saturation with 10 pulses of an electric field at 25 kV/cm (Barbosa-Cánovas and others 1999).
Critical treatment time also depends on the electric field intensity applied. Above the critical electric field, critical treatment time decreases with higher electric fields. Barbosa-Cánovas and others (1999) reported that for an electric field strength 1.5 times higher than Ec, the critical treatment time would remain constant.
c) Pulse waveshape. Electric field pulses may be applied in the form of exponential decaying, square-wave, oscillatory, bipolar, or instant reverse charges. Oscillatory pulses are the least efficient for microbial inactivation, and square wave pulses are more energy and lethally efficient than exponential decaying pulses. Bipolar pulses are more lethal than monopolar pulses because a PEF causes movement of charged molecules in the cell membranes of microorganisms, and reversal in the orientation or polarity of the electric field causes a corresponding change in the direction of charged molecules (Ho and others 1995; Qin and others 1994). This difference was reported in Bacillus spp. spores (Ho and Mittal 1997) and E. coli (Qin and others 1994). With bipolar pulses, the alternating changes in the movement of charged molecules cause a stress in the cell membrane and enhance its electric breakdown. Bipolar pulses also offer the advantages of minimum energy utilization, reduced deposition of solids on the electrode surface, and decreased food electrolysis (Barbosa-Cánovas and others 1999).
As mentioned earlier in this report, the instant-charge-reversal pulse can be described as partially positive at first and partially negative immediately thereafter. This characteristic of the waveshape is influenced by the electrical conductivity of the treated food. In this regard, an increase in conductivity decreases the duration of the positive part of the pulse as well as the span of the negative part, which in turn increases the overall peak/voltage ratio.
The difference between a bipolar and instant charge reverse pulse is the relaxation time between pulses, which is only present in the former. The inactivation effect of an instant-reversal-charge is believed to be due to a significant alternating stress on the microbial cell that causes structural fatigue. Ho and Mittal (1997) reported that instant-reversal-charge may reduce the critical electric field strength required for electroporation of the microbial cell. The effectiveness of this waveform to inactivate microorganisms compared to other pulse waveforms can save up to 1/5 or 1/6 of total energy and equipment cost. Further work is required to verify the effect of reversal-charge pulses on the inactivation ratio. The inactivation of Bacillus subtilis and Bacillus cereus spores suspended in NaCl solutions has been reported to be higher when instant reverse pulses and a polarity of electric field chambers with high pulse frequencies are used. Instant reverse charge has been reported to be effective in inactivation of 5-log cycles of Bacillus spp. spores. These researchers established that the survival fraction is not only a function of the temporal pulse area but that even when both bipolar (alternating exponential) and exponential waves had the same area per pulse, bipolar waves yielded a higher inactivation ratio (Ho and Mittal 1997).
A study conducted by Zhang and others (1997) showed the effect of square wave, exponentially decaying, and instant-charge-reversal pulses on the shelf-life of orange juice. Three waveshape pulses were used: (a) square waves with peak electric field of 35 kV/cm, an effective pulse width of 37.22 µs, and a pulse rise time of 60 ns; (b) exponential decaying waves with a peak electric field of 62.5 kV/cm, an effective pulse width of 0.57 µs and a pulse rise time of 40 ns; and (c) charge-reversal waves with a peak electric field of 37 kV/cm, an effective pulse width of 0.96 µs, and a pulse rise of 400 ns. Square wave pulses were more effective, yielding products with longer shelf-lives than those products treated with exponentially decaying and charge reverse pulses. In agreement with this study, Love (1998) quantitatively demonstrated the stronger inactivation effect of square wave pulses over other wave shapes.
Qin and others (1994) studied the inactivation of S. cerevisiae using square and exponential decay waveforms and a peak electric field of 12 kV/cm and 60 J/pulse for both waveforms. The results of this investigation suggested that both waveforms were effective in the microbial inactivation, with square wave pulse waveform being the most effective.
d) Treatment temperature. Experimental results have demonstrated that, in challenge tests, both treatment temperatures and process temperatures impact microbial survival and recovery.
PEF treatments at moderate temperatures (~ 50 to 60 ° C) have been shown to exhibit synergistic effects on the inactivation of microorganisms (Jayaram and others 1992; Dunn and Pearlman 1987). With constant electric field strength, inactivation increases with an increase in temperature. Because the application of electric field intensity does cause some increase in the temperature of the foods, proper cooling is necessary to maintain food temperatures far below those generated by thermal pasteurization.
The effect of temperature was observed when E. coli reduction increased from 1 to 6.5-log reduction cycles with a temperature change from 32 to 55 ° C (Vega-Mercado and others 1996a). A higher lethal effect of PEF treatment is accomplished by increasing the process temperature to 25 ° C, from 5 or 10 ° C. This may be due to the increase in the electrical conductivity of the solution at the higher temperature (Marquez and others 1997). The authors suggested that the leakage of mobile ions in decoated spores may increase as the temperature is raised due to an increase in average kinetic energy of the ions. A higher temperature also increases the motion of the solvent molecules in both the surrounding cortex and the core so that the molecules could migrate from one electrode to the other.
Additional effects of high treatment temperatures are changes in cell membrane fluidity and permeability, which increases the susceptibility of the to cell to mechanical disruption (Hulsheger and others 1981). Also, a low transmembrane potential decreases Ec and therefore increases inactivation (Jeyamkondan 1999).
2.1.2. Product factors
a) Conductivity, pH, and ionic strength. The electrical conductivity of a medium (σ , Siems/m), which is defined as the ability to conduct electric current, is an important variable in PEF. Electrical conductivity is the inverse of the resistivity, which is defined by the letter r and measured in ohm-meters (W .m). Foods with large electrical conductivities generate smaller peak electric fields across the treatment chamber and therefore are not feasible for PEF treatment (Barbosa-Cánovas and others 1999). Inactivation of Lactobacillus brevis with PEF showed that as the conductivity of the fluid increased, the resistance of the treatment chamber was reduced (Jayaram and others 1992), which in turn reduced the pulse width and decreased the rate of inactivation. Because an increase in conductivity results from increases the ionic strength of a liquid, an increase in the ionic strength of a food results in a decrease in the inactivation rate. Furthermore, an increase in the difference between the conductivity of a medium and microbial cytoplasm weakens the membrane structure due to an increased flow ionic substance across the membrane. Thus, the inactivation rate of microorganisms increases with decreasing conductivity even with an application of equal input energy (Jayaram and others 1992). Another study by Dunne and others (1996) with a model system showed resistivity had no effect on PEF effectiveness on E. coli and L. innocua. These apparent controversial results may be due to the microorganisms or media used.
Vega-Mercado and others (1996b) studied the effect of pH and ionic strength of the medium (SMFU) during PEF treatment. The inactivation ratio increases from not detectable to 2.5-log cycles when ionic strength solutions were adjusted from 168 to 28mM. At 55 kV/cm (8 pulses), as the pH was reduced from 6.8 to 5.7, the inactivation ratio increased from 1.45- to 2.22-log cycles. The PEF treatment and ionic strength were responsible for electroporation and compression of the cell membrane, whereas the pH of the medium affected the cytoplasm when the electroporation was complete. Dunne and others (1996) reported that, depending on the microorganism, acidic pH enhanced microbial inactivation. No mention was made of what microorganisms were affected or what range of pH was used.
b) Particulate foods. Inactivation of microorganisms in liquid-particulate systems has been studied by Dunne et at (1996). E. coli, L. innocua, Staphyloccocus aureus, and Lactobacillus acidophilus were suspended in a 2 mm diameter alginate beads model, and the effect of variables in PEF microbial inactivation was tested. The researchers concluded that the process was effective in killing microorganisms embedded in particulates. However, to achieve more than a 5-log cycle reduction, high energy inputs were needed (70 - 100 J/ml, depending on the bacteria). With those high PEF intensities, the possibility of dielectric breakdown exists- a limitation still to be overcome. Qin and others (1995c) reported that dielectric breakdown occurs when air or liquid vapor is present in the food because of the difference in dielectric constant between liquid and gas. Likewise, dielectric breakdown may occur at a particle- to -liquid interface due to differences in electric constants.
c) Hurdle approach. In general, the combination of factors (hurdles) such as pH, ionic strength and antimicrobial compounds during PEF treatment would be an effective means to aid in the inactivation of microorganisms with PEF.
2.1.3. Microbial factors
a) Type of microorganisms. Among bacteria, those that are gram-positive are more resistant to PEF than those that are gram-negative (Hülsheger and others 1983). In general, yeasts are more sensitive to electric fields than bacteria due to their larger size, although at low electric fields they seem to be more resistant than gram-negative cells (Sale and Hamilton 1967; Qin and others 1995a). A comparison between the inactivation of 2 yeast spp. of different sizes showed that the field intensity needed to achieve the same inactivation level was inversely proportional to cell size. Those results are logical but inconsistent with results by Hülsheger and others (1983). Studies need to continue in this area to better understand the effect of the type of microorganism on microbial inactivation.
b) Concentration of microorganisms. The number of microorganisms in food may have an effect on their inactivation with electric fields. Barbosa-Cánovas and others (1999) reported that inactivation of E. coli in a model food system of simulated milk ultrafiltrate (SMUF) was not affected when the concentration of microorganisms was varied from 103 to 108 cfu/ml after being subjected to 70 kV/cm, 16 pulses, and a pulse width of 2 µs. Increasing the number of S. cerevisiae in apple juice resulted in slightly lower inactivation (25 kV/cm, 1 pulse, and pulse width of 25 µs). The effect of microbial concentration on inactivation may be related to cluster formation of yeast cells or possibly concealed microorganisms in low electric field regions.
c) Growth stage of microorganisms. In general, logarithmic phase cells are more sensitive to stress than lag and stationary phase cells. Microbial growth in logarithmic phase is characterized by a high proportion of cells undergoing division, during which the cell membrane is more susceptible to the applied electric field. Hülsheger and others (1983) concluded that cells from logarithm growth phase are more sensitive to PEF than from the stationary growth phase. Likewise, E. coli cells in the logarithmic phase were more sensitive to PEF treatment when compared to cells in the stationary and lag phases (Pothakamury and others 1996). Studies with S. cerevisiae have shown that the susceptibility of actively growing cells to PEF also occurs with yeast cells (Jacob and others 1981; Gaskova and others 1996). For instance, Gaskova and others (1996) reported that the killing effect of PEF in the logarithmic phase is 30% greater than of those in stationary phase of growth.
2.2. Data from Microbial Inactivation Studies
Numerous publications on inactivation present data on vegetative cells, the majority of them from a few genera. Tables 1, 2, and 3 summarize research on the inactivation of microorganisms and enzymes. Table 1 lists the published papers on microorganisms and enzymes, except for E. coli and S. cerevisiae. Tables 2 and 3 list inactivation data collected from S. cerevisiae and E. coli, respectively. The tables include, when available, information on the treatment vessel, process conditions (treatment time, temperature, electric field intensity, number of pulses, and waveshape), media, and data on the log reduction achieved.
Various inactivation levels of S. cerevisiae have been achieved in food models and foods using a variety of PEF chambers and experimental conditions (Mizuno and Hori 1991; Zhang and others 1994a, 1994b; Qin and others 1994, 1995a). Other yeasts of importance in food spoilage have also been reduced, suggesting PEF's potential to prevente or delay yeast-related food spoilage.
Fernandez-Molina and others (1999) reported 2.6- and 2.7-log reductions for different microorganisms such as L. innoccua and Pseudomonas fluorescens with 2 μs 100 pulses at 50 kV/cm at ambient temperature. The influence of the food composition was shown by Calderon-Miranda (1998) studies where L. innoccua was reduced by 2.4- and 3.4-log cycle reductions in raw skim milk and liquid whole egg, respectively, under the same experimental conditions.
Hülsheger and others (1983) tested PEF inactivation effectiveness of a variety of microorganisms in phosphate buffer, under the same conditions. The results from these studies suggested that L. monocytogenes (2-log reduction) is more resistant to PEF than Pseudomonas auruginosa or S. aureus (3- to 3.5-log reduction cycles), and that Candida albicans was the most sensitive microorganism among them (4.5-log reduction cycle). For these experiments 30 pulses of 36 μs duration of 20 kV/cm were applied.
Grahl and others (1992) reported the influence of pulse number in microbial inactivation of E. coli. They were able to reduce populations of E. coli in UHT milk by 1-, 2-, and 3-log cycles when 5, 10, and 15 pulses (22 kV/cm) were applied. Qin and others (1998) achieved more than a 6-log cycle reduction in E. coli suspended in simulated milk ultrafiltrate (SMUF) using electric field intensity of 36 kV/cm with a 5-step (50 pulses) PEF treatment. The temperature in the chamber was maintained below 40 ° C during the PEF treatment, which is lower than the temperature of commercial pasteurization (70 to 90 ° C) for milk. Hülsheger and others (1983) reported a 4-log reduction of E. coli in an electric field intensity of 40 kV/cm accompanied with a long treatment time of 1080 μs. A PEF method suitable to inactivate up to 7-log cycles of E. coli with fewer pulses (20 versus 70) is stepwise recirculation whereby the product is processed several consecutive times (Barbosa-Cánovas and others 1999). Liu and others (1997) reported that PEF and organic acids (benzoic and sorbic) achieved 5.6- and 4.2-log cycle reductions, compared to a 1-log cycle reduction when PEF was used alone, suggesting enhanced effects with the combination of PEF and organic acids.
The higher efficiency of bipolar pulses versus monopolar pulses was suggested by Qin and others (1994). Cells of B. subtilis were reduced to 3- and <2-log cycles when bipolar and monopolar pulses were applied, respectively.
Inactivation studies on the effects of PEF on bacterial spores are scarce and results vary. Early studies (Sale and Hamilton 1967) reported that Bacillus spp. spores were resistant to exponential wave PEF with strength fields up to 30 kV/cm. Only after germination did they become sensitive to PEF. Simpson and others (1995) confirmed the high resistance of B. subtilis spores to PEF, and subsequently studied a hurdle approach with heat-shock, lysozyme, EDTA, and pH. Only a combination of 80 ° C heat-shock, lysozyme, followed by PEF at 60 ° C was able to achieve a 2- to 4-log cycle reduction of spores. The resistance of spores to PEF was shown by Pothakamury (1995). They reported only 3- to 4-log reduction cycles for B. subtilis ATCC 9372 spores that were subjected to 60 pulses of 16 kV/cm electric field intensity and 200 - 300 μs pulse widths. Pagán and others (1998) found that spores of B. subtilis were not inactivated when PEF (60 kV/cm, 75 pulses) was used in combination with high hydrostatic pressure (HHP) (1500 atm, 30 min, 40 ° C). These treatments, however, induced the germination of the spores of B. subtilis by more than 5-log cycles, making them sensitive to subsequent pasteurization heat treatment. Thus, combinations of HHP, PEF, and heat treatments constitute an alternative to the stabilization of food products by heat to inactivate spores. Marquez and others (1997) successfully inactivated 3.4- and 5-log cycles of B. subtilis and B. cereus spores at room temperature, an electric field of 50 kV/cm, and 30 and 50 instant-charge-reversal pulses, respectively.
As Tables 1, 2, and 3 show, many researches have studied the effects of pulsed electric fields in microbial inactivation; however, due to the numerous critical process factors and broad experimental conditions used, definite conclusions about critical process factors effects on specific pathogen reductions cannot be made. Research that provides conclusive data on the PEF inactivation of pathogens of concern is clearly needed.
3. Mechanisms of Microbial Inactivation
3.1. Analysis of Microbial Inactivation Mechanism (s)
Two mechanisms have been proposed as the mode of action of PEF on microorganisms: electrical breakdown and electroporation.
3.1.1. Electrical breakdown
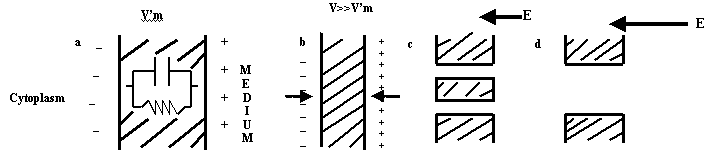
Zimmermann (1986), as shown in Fig. 5, explains what electrical breakdown of cell membrane entails. The membrane can be considered as a capacitor filled with a dielectric (Fig. 5a). The normal resisting potential difference across the membrane V'm is 10 mV and leads to the build-up of a membrane potential difference V due to charge separation across the membrane. V is proportional to the field strength E and radius of the cell. The increase in the membrane potential leads to reduction in the cell membrane thickness. Breakdown of the membrane occurs if the critical breakdown voltage Vc (on the order of 1 V) is reached by a further increase in the external field strength (Fig. 5c). It is assumed that breakdown causes the formation of transmembrane pores (filled with conductive solution), which leads to an immediate discharge at the membrane and thus decomposition of the membrane. Breakdown is reversible if the product pores are small in relation to the total membrane surface. Above critical field strengths and with long exposure times, larger areas of the membrane are subjected to breakdown (Fig. 5d). If the size and number of pores become large in relation to the total membrane surface, reversible breakdown turns into irreversible breakdown, which is associated with mechanical destruction of the cell membrane.
The corresponding electric field is Ecritical = Vcritical /fa, where a is the radius of the cell and f is a form that depends on the shape of the cell (Schoenbach and others 1997). For a spherical cell, f is 1.5; for cylindrical cells of length l and hemispheres of diameter d at each end, the form factor is f = l(l - d)/3. Typical values of Vcritical required for the lysing of E. coli are on the order of 1 V. The critical field strength for the lysing of bacteria with a dimension of approximately 1 µm and critical voltage of 1 V across the cell membrane is therefore on the order of 10 kV/cm for pulses of 10 microsecond to millisecond duration (Schoenbach and others 1997).
|
| Figure 5. Schematic diagram of reversible and irreversible breakdown. (a) cell membrane with potential V'm, (b) membrane compression, (c) pore formation with reversible breakdown, (d) large area of the membrane subjected to irreversible breakdown with large pores (Zimmermann, 1986) |
3.1.2. Electroporation
Electroporation is the phenomenon in which a cell exposed to high voltage electric field pulses temporarily destabilizes the lipid bilayer and proteins of cell membranes (Castro and others 1993). The plasma membranes of cells become permeable to small molecules after being exposed to an electric field, and permeation then causes swelling and eventual rupture of the cell membrane (Fig. 6) (Vega-Mercado 1996b). The main effect of an electric field on a microorganism cell is to increase membrane permeability due to membrane compression and poration (Vega-Mercado and others 1996b). Kinosita and Tsong (1977; 1979) demonstrated that an electric field of 2.2 kV/cm induced pores in human erythrocytes of approximately 1 nm in diameter. Kinosita and Tsong (1977) suggested a 2-step mechanism for pore formation in which the initial perforation is a response to an electrical suprathreshold potential followed by a time-dependent expansion of the pore size (Fig. 6). Large pores are obtained by increasing the intensity of the electric field and pulse duration or reducing the ionic strength of the medium.
In a lipid model vesicle (liposome), the electrophoretic movement of ions and water dipoles through the spontaneous hydrophobic pores is postulated to be the first event of electroporation, after which lipid molecules rearrange to form more stable hydrophilic pores. This could also take place in a cell membrane. In addition, protein channels, pores, and pumps in these membranes are extremely sensitive to transmembrane electric field and become initiation sites for the electropores (Tsong 1990). In the cell membrane charges to electric dipoles of lipids, proteins, carbohydrates, and ions and the polarizability of these molecules make up the electric field. Therefore, electroporation occurs both in the liposomes and cell membranes, but the molecules affected by the applied field are not necessarily the same in these 2 systems (Tsong 1990). The gating potentials to the channel constituted by the proteins are in the 50 - mV range (Castro and others 1993).
Miller and others (1988) found that electroporation permits the uptake of DNA by mammalian cells and plant protoplasts because it induces transient permeability to the cell membrane. These researchers demonstrated the utility of high-voltage electroporation for the genetic transformation of intact bacterial cells by using the enteric pathogen Campylobacter jejuni as a model system. The method involved the exposure of a C. jejuni cell suspension to a high-voltage potential decay discharge of 5 - 13 kV/cm with a short treatment time ranging between 2.4 - 2.6 µs in the presence of plasmid DNA. Electrical transformation of C. jejuni resulted in frequencies as high as 1.2 x 106 transformats per µg of DNA.
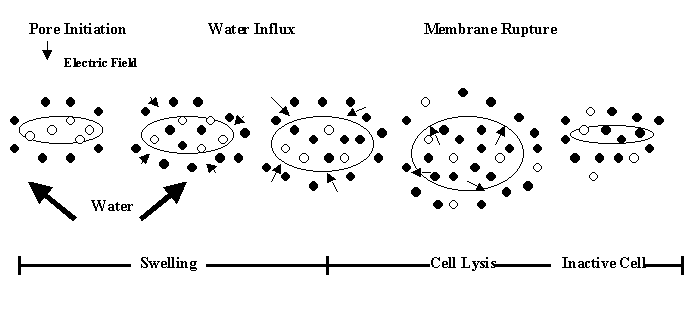 |
| Figure 6. Electroporation of a cell membrane (Vega-Mercado, 1996b) |
3.2. Inactivation Models
Hülsheger and Niemann (1980) were the first to propose a mathematical model for inactivation of microorganisms with PEF. Their model was based on the dependence of the survival ratio S (N/No or the ratio of living cell count before and after PEF treatment) on the electric field intensity E according to the following expression:
ln(S) = -bE(E-Ec) (1)
where bE is the regression coefficient, E is the applied electric field, and Ec is the critical electric field obtained by the extrapolated value of E for 100% survival. The regression coefficient describes the gradient of the straight survival curves and is a microorganism-media constant. The critical electric field (Ec) has been found to be a function of the cell size (much lower for large cells) and pulse width (that is, with pulse width > 50 µs, Ec = 4.9 kV/cm; pulse width > 2 µs, Ec = 40 kV/cm). Hülsheger and others (1981) proposed an inactivation kinetic model that relates microbial survival fraction (S) with PEF treatment time (t) in the form of
where bt is the regression coefficient, t is the treatment time, and tc is the extrapolate value of t for 100% survival, or critical treatment time. The model can also be expressed as
 (3)
(3)
where t is treatment time, tc is critical treatment time, Ec is critical electric field intensity, and K is the kinetic constant. Table 4 shows K values calculated by fitting experimental data for the cited microorganisms (Hülsheger 1983). A small value for the kinetic constant [K] indicates a wide span in the inactivation rate curve and lower sensitivity to PEF, whereas a large value implies a steep decline or higher susceptibility to PEF. Lower Ec values would indicate less resistance to the PEF treatment.
Table 4 shows that Ec for gram-negative bacteria is lower than that for gram-positive, in accordance with the smaller PEF resistance of the former. The kinetic constant for the yeast C. albicans is smaller than for gram-negative and gram-positive bacteria, implying that yeast are more resistant to inactivation with PEF than bacteria. This result is inconsistent with results from other studies. The table also shows that E. coli cells in the log stage of growth have lower tc and Ec and higher K than cells, which is in accordance with other studies. Correlation coefficients of the lines where high, indicating the model may have some future use.
A second model proposed by Peleg (1995) describes a sigmoid shape of the survival curves generated by the microbial inactivation with PEF. The model (equation 4) represents the percentage of surviving organisms as a function of the electric fields and number of pulses applied. This model is defined by a critical electric field intensity that corresponds to 50% survival (Ed) and a kinetic constant (Kn, a function of the number of pulses) that represents the steepness of the sigmoid curve:
Mathematically, about 90% inactivation is achieved within the critical electric field plus 3 times the kinetic constant. In this generalized model, Ed(n) and K(n) are algebraic functions that not only depend on the electric field but also on the number of pulses or treatment time. The
 (5)
(5)
model can be simplified by not considering the relationship between the electric field and the number of pulses:
A small value for the kinetic constant [K (n) or K] indicates a wide span in the inactivation rate curve and lower sensitivity to PEF, whereas a large value implies a steep decline or higher susceptibility to PEF. Lower Ed values would indicate less resistance to the PEF treatment.
Table 5 shows the kinetic constant for various microorganisms calculated using Peleg's equation. Experimental data was compiled from various published studies performed with those microorganisms and were fitted to the Peleg's model (Peleg 1995). Results indicate that the higher the number of pulses, the lower the Ed and kinetic constant K. The high regression coefficients for all the studies show the model has potential use to predict microbial inactivation.
| Table 4. Kinetic constants of Hülshelger's model for different microorganisms suspended in a Na2HPO4/KH2PO4 buffer with pH of 7.0. | |||||||
 |
|||||||
| Microorganism | E | t | Ec | tc | K | r | |
| (kV/cm) | (µs) (kV/cm) | (µs) | (kV/cm) | (%) | |||
| Escherichia coli (4 h)1 | 4 - 20 | 0.07 - 1.1 | 0.7 | 11 | 8.1 | 97.7 | |
| E.coli (30 h)1 | 10 - 20 | 0.07 - 1.1 | 8.3 | 18 | 6.3 | 97.6 | |
| Klebsiella pneumonia | 8 - 20 | 0.07 - 1.1 | 7.2 | 29 | 6.6 | 95.7 | |
| Pseudomonas auriginosa | 8 - 20 | 0.07 - 1.1 | 6.0 | 35 | 6.3 | 98.4 | |
| Staphylococcus aureus | 14 - 20 | 0.07 - 1.1 | 13.0 | 58 | 2.6 | 97.7 | |
| Listeria monocytogenes I | 12 - 20 | 0.07 - 1.1 | 10.0 | 63 | 6.5 | 97.2 | |
| L. monocytogenes II | 10 - 20 | 0.07 - 1.1 | 8.7 | 36 | 6.4 | 98.5 | |
| Candida albicans | 10 - 20 | 0.14 - 1.1 | 8.4 | 110 | 2.2 | 96.6 | |
| (From Hulsheger and others 1983)
E, electric field; t, treatment time; Ec, critical electric field; tc, critical time; K, kinetic constant; r, correlation coefficient of regression line; 1Incubation time. |
|||||||
| Table 5 Kinetic Constants of Peleg's model. | |||||
| Organism | Number of Pulses | Ed (kV/cm) |
K (kV/cm) |
r2 | |
| Lactobacillus brevis | - | 11.4 | 1.6 | 0.973 | |
| Saccaromyces cerevisiae | - | 13.2 | 2.3 | 0.994 | |
| Staphylococcus aureus | - | 14.1 | 2.0 | 0.991 | |
| Candida albicans | 2 | 21.2 | 3.1 | 0.999 | |
| 4 | 15.3 | 3.1 | 0.993 | ||
| 10 | 10.1 | 1.3 | 0.997 | ||
| 30 | 7.5 | 1.2 | 0.999 | ||
| Listeria monocytogenes | 2 | 14.9 | 2.8 | 0.981 | |
| 4 | 12.7 | 2.0 | 0.994 | ||
| 10 | 10.3 | 2.4 | 0.99 | ||
| 30 | 8.5 | 2.0 | 0.999 | ||
| Pseudomonas aeruginosa | 2 | 12.9 | 2.6 | 0.982 | |
| 4 | 10.6 | 2.4 | 0.994 | ||
| 10 | 8.3 | 2.1 | 0.99 | ||
| 30 | 6.7 | 1.8 | 0.999 | ||
| (from Peleg 1995) Ed, electrical field when 50% of population is reduced; K, kinetic constant; r2, regression coefficient |
|||||
4. Validation/Critical Process Factors
4.1. Summary of Critical Process Factors
Extensive microbial inactivation tests have been conducted to validate the concept of PEF as a non-thermal food pasteurization process (Zhang and others 1994a, 1994b; Zhang and others 1995a, 1995b; Pothakamury and others 1995; Keith and others 1996, Marquez and others 1997; Qin and others 1995a, 1995b. 1995c; Vega-Mercado and others 1996a; 1996b; Qin and others 1998; Castro and others 1993).
High intensive pulsed electric field treatments produce a series of degradative changes in blood, algae, bacteria and yeast cells (Castro and others 1993). The changes include electroporation and disruption of semipermeable membranes which lead to cell swelling and/or shrinking, and finally to lysis of the cell. The mechanisms for the inactivation of microorganisms include electric breakdown, ionic punch-through effect, and electroporation of cell membranes (Qin and others 1994). The inactivation of microorganisms is caused mainly by an increase in their membrane permeability due to compression and poration (Vega-Mercado and others 1996b).
Castro and others (1993) reported a 5-log reduction in bacteria, yeast, and mold counts suspended in milk, yogurt, orange juice and liquid egg treated with PEF. Zhang and others (1995a) achieved a 9-log reduction in E. coli suspended in simulated milk ultrafiltrate (SMUF) and treated with PEF by applying a converged electric field strength of 70 KV/cm and a short treatment time of 160 µs. This processing condition is adequate for commercial food pasteurization that requires 6- to 7-log reduction cycles (Zhang and others 1995a).
In conclusion, numerous critical process factors exist. Carefully designed studies need to be performed to better understand how these factors affect populations of pathogens of concern.
4.2. Methods to Measure Critical Process Factors
PEF critical process factors may be monitored as follows:
- Pulse voltage waveform. The average electric field strength is calculated by dividing the peak voltage by the gap distance between the electrodes. A voltage probe and an oscilloscope make such measurement. Data logging is necessary to keep this critical process variable.
- Pulse current waveform. Pulse current should have a waveform very similar to that of the voltage waveform, different by a ratio, the load resistance. In the case of a partial breakdown, the ratio changes. A shunt resistor or a current monitor, such as a Pearson Coil, together with an oscilloscope may be used to measure the current waveform.
- Pulse duration time is determined from the voltage waveform.
- Pulse repetition rate.
- Voltage waveform, current waveform, duration time, and repetition rate may be logged by a computerized oscilloscope system.
- Temperatures at the inlet and outlet of each treatment chamber should be monitored. A Resistive Temperature Device (RTD) may be used on-line for such monitors. Temperature data may be used to estimate the energy delivery to the PEF chamber.
- Flow rate should be monitored because it determines the resident time within a treatment chamber, allowing the number of pulses applied to be determined.
In some continuous PEF processing systems, pressure should also be monitored. An on-line pressure transmittor may be used for this purpose.
4.3. Microbial Surrogates
Currently, there is no information on the use of surrogate microorganisms as indicators of pathogenic bacteria when PEF is used as a processing method. Selection of surrogates will require the prior identification of the microorganism of concern in a specific food and PEF system. In PEF, as with other inactivation methods, the potential for injury and recovery exists. Experts should consider this possibility and choose the appropriate microbial enumeration methods. The selection of the appropriate surrogate(s) will depend on the type of food, microflora, and process conditions (that is, electric field intensity, number of pulses, treatment time, pulse wave) and should also follow the general guidelines listed in the Overarching Principles.
S. cerevisiae and Candida spp. are 2 microorganisms of particular relevance in spoilage of foods. Although their inactivation has been proven in many food models and foods, their susceptibility to PEF may prevent their use as a surrogate.
5. Process Deviations
5.1. Methods for Determining Process Deviations
Continuous monitoring of storage temperatures, pH, color, and acidity of PEF-treated and -untreated products will indicate any deviation of products from their standardized conditions. A data acquisition system is needed to monitor the number of pulses and the frequency applied to the food products. A digital oscilloscope is required to monitor the wave shape and the peak electric field. To ensure desirable temperature during PEF processing of foods, digital thermocouples or fiber optic probes must be used to record the temperature the entrance and exit of the PEF treatment chamber.
5.2. Methods to Assess Deviation Severity
5.2.1.Temperature sensors
Temperature sensors such as thermocouples are connected from the tubing at the entrance and exit of the PEF treatment chamber. A continuous recording of temperature will avoid undesirable temperature increases caused by overheating treatment electrodes inside the chamber.
5.2.2. Data acquisition system
A computer with data acquisition systems will monitor the entire system. Continuous recording of the number of pulses and frequencies will correct such deviations caused by malfunction of the high voltage power supply, which may lead to underprocessed product.
5.2.3. Automatic shut downAborting the pulser automatically from the computer will avoid damage to the chamber and electrode due to arcing. If there is no product leakage, the equipment can be restarted and the product can be reprocessed. Otherwise, it has to be discarded.
5.2.4. Sample deviation
Milk is a fluid containing proteins and minerals, such as calcium, iron, and magnesium, that are very likely to cause fouling on the electrode surface during PEF treatment. If the milk has a high level of microorganisms, this film may serve as a good substrate for microorganisms to reproduce and form a biofilm in the treatment chamber. Therefore, the efficiency of the pulser is lower and the milk will receive fewer pulses due to the clotting on the electrodes. To resolve this situation, and in order to attain the required processing conditions, optimization of the process has to be performed.
6. Research Needs
Despite significant developments in PEF technologies in the 1990s several areas need further research before the technology is applied commercially. These include:
- Confirming the mechanisms of microbial and enzyme inactivation.
- Identification of the pathogens of concern most resistant to PEF.
- Identification of surrogate microorganisms for the pathogens of concern.
- Development of validation methods to ensure microbiological effectiveness.
- Development and evaluation of kinetic models that take into consideration the critical factors influencing inactivation.
- Studies to optimize and control critical process factors.
- Standardization and development of effective methods for monitoring consistent delivery of a specified treatment.
- Treatment chamber design uniformity and processing capacity.
- Identification and application of electrode materials for longer operation time and lower metal migration.
- Process system design, evaluation, and cost reduction.
Glossary
A complete list of definitions regarding all the technologies is located at the end of this document.
Batch or static chamber. Chamber that treats a static mass of food in bulk or packaged. A chamber that processes a limited volume of food at one time.
Breakdown. Rupture of bacterial cell membranes with the application of an electric field
Capacitor bank. Network of 2 or more capacitors used to store the energy supply from a DC power source.
Co field flow. One possible configuration for a PEF continuous chamber
DC power supply. Electric device to deliver direct current to the capacitor bank.
Continuous chamber. Opposite to batch chamber, it processes liquid foods that are pumped between pulsing electrodes.
Electric field intensity or strength Average voltage (kV) divided by the distance between 2 electrodes (cm).
Electrical breakdown. An abrupt rise in electric current in the presence of a small increase in voltage. As a consequence, rupture of bacterial cell membranes may occur with the application of an electric field. This effect is more pronounced in pulsed electric field treatment. In microwaves, this can happen if operating at very low pressures, as in freeze-drying.
Electrical conductivity. Physical property of a food material that determines its ability to conduct electricity, expressed in Siemens per cm (S/cm).
Electroporation. Destabilization of the lipid bilayer and proteins of cell membranes, as well as the formation of pores induced when a microbial cell is temporarily exposed to high voltage electric field pulses.
Electrode gap. Distance (cm) between the inner and outer electrode inside PEF treatment chambers.
Input voltage. Voltage (kV) supplied from a DC power source.
Irreversible breakdown. Irreversible generation of pores in the bacterial cell membranes.
Peak voltage. Maximum voltage (kV) delivered by PEF system.
Pulse width or time constant. Duration of the pulse. For an exponential decaying pulse, the
resistance of the food times the capacitor capacitance gives a measure of the pulse width.
Pulse rate. Number of pulses per s or input frequency (1/s).
Reversible breakdown. Formation of reversible pores in the bacterial cell membranes.
Treatment time. The product of the number of pulses and the duration of the pulses, usually expressed in microseconds (µs).
Waveform/Waveshape. Type of electric pulses generated by the high-voltage pulser.
REFERENCES
Barbosa-Cánovas, G. V., Gongora-Nieto, M. M., Pothakamury, U. R., Swanson, B. G. 1999. Preservation of foods with pulsed electric fields. 1-9, 76-107, 108-155. Academic Press Ltd. London.
Calderon-Miranda, M. L. 1998. Inactivation of listeria inocua by pulsed electric fields and nisin. Pullman, WA. Washington State University.
Castro, A. J., Barbosa-Cánovas, G. V. and Swanson, B. G. 1993. Microbial inactivation of foods by pulsed electric fields. J Food Process Pres. 17:47-73
Castro, A. J. 1994. Pulsed electrical field modification of activity and denaturation of alkaline phosphatase. Food Science and Human Nutrition. Pullman, WA. Washington State University.
Dunn, J. E. and Pearlman, J. S. 1987. Methods and apparatus for extending the shelf-life of fluid food products. Maxwell Laboratories, Inc. U. S. Patent 4,695,472.
Dunn, J. 1996. Pulsed light and pulsed electric field for foods and eggs. Poul Sci. 75(9):1133-1136
Dunne, C. P., Dunn, J., Clark, W., Ott, T. and Bushnell, A. H. 1996. Application of high energy electric field pulses to preservation of foods for combat rations. Science and Technology for Force XXI. Department of the Army. Norfolk, Virginia. June 24-27. 7.
EPRI. 1998. Pulsed electric field processing in the food industry: a status report on PEF. Palo Alto, CA. Industrial and Agricultural Technologies and Services. CR-109742.
Fernandez-Molina, J. J., Barkstrom, E., Torstensson, P., Barbosa-Cánovas, G. V. and Swanson, B. G. 1999. Shelf-life extension of raw skim milk by combining heat and pulsed electric fields. Food Res Int.
Gaskova, D., Sigler, K., Janderova, B. and Plasek, J. 1996. Effeck of high-voltage electric pulses on yeast cells: Factors influencing the killing efficiency. Bioelectrochem Bioenergetics. 39:195-202
Grahl, T., Sitzmann, W. and Markl, H. 1992. Killing of microorganisms in fluid media by high-voltage pulses. DECHEMA Biotechnology Conferences. 675-679.
Grahl, T. and Maerkl, H. 1996. Killing of microorganisms by pulsed electric fields. Applied MicrobiolBiotechnol. 45(1/2):148-157
Gupta, R. P. and Murray, W. 1988. Pulsed high electric field sterilization. IEEE Pulsed Power Conf. Record. National Research Council. 58-64.
Ho, S. Y., G.S., M., Cross, J. D. and Griffiths, M. W. 1995. Inactivation of Pseudomonas fluorescens by high voltage electric pulses. J Food Sci. 60(6):1337-1343
Ho, S. Y., Mittal, G. S. and Cross, J. D. 1997. Effects of high field electric pulses on the activity of selected enzymes. J Food Eng. 31(1):69-84
Ho, S. Y. and Mittal, G. S. 1997. Analysis of 2 high voltage electric pulse systems for batch and continuous pasteurization of selected food products. Universty of Guelph. confidential.
Hülsheger, H. and Nieman, E. G. 1980. Lethal effect of high-voltage pulses on e. coli K12. Radiat Environ Biophys 18(4):281-8
Hülsheger, H., Pottel, J. and Niemann, E. G. 1981. Killing of bacteria with electric pulses of high field strength. Radiat Environ Biophys. 20:53-65
Hülsheger, H., Pottel, J. and Niemann, E. G. 1983. Electric field effects on bacteria and yeast cells. Radiat Environ Biophys. 22:149-162
Jacob, H. E., Forster, W. and Berg, H. 1981. Microbial implications of electric field effects. II. Inactivation of yeast cells and repair of their cell envelope. Z. Allg. Microbial 21(3):225-232
Jayaram, S., Castle, G. S. P. and Margaritis, A. 1992. Kinetics of sterilization of Lactobacillus brevis cells by the application of high voltage pulses. Biotechnol Bioeng. 40(11):1412-1420
Jeyamkondan, S., Jayas, D. S. and Holley, R. A. 1999. Pulsed electric field processing of foods: a review. J Food Protect. 62(9):1088-1096
Keith, W. D., Harris, L. J., Hudson, L. and Griffiths, M. 1997. Pulsed electric fields as a processing alternative for microbial reduction in spice. Food Res Int. 30(3/4):185-191
Kinosita, K. J. and Tsong, T. Y. 1977. Voltage induced pore formation and haemolysis erythrocytes. Biochim Biophys Acta. 471:227-242
Kinosita, K. J. and Tsong, T. Y. 1979. Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta. 554:479-497
Knorr, D., Geulen, M., Grahl, T. and Sitzmann, W. 1994. Food application of high electric field pulses. Trends Food Sci Technol. 5:71-75
Liu, X., Yousef, A. E. and Chism, G. W. 1997. Inactivation of Escherichia coli O157:H7 by the combination of organic acids and pulsed electric field. J Food Safety. 16(4):287-299
Love, P. 1998. Correlation of fourier transforms of pulsed electric field waveform and microorganism inactivation. IEEE Transactions on Dielectrics and Electrical Insulation. 5(1):142-147
Lubicki, P. and Jayaram, S. 1997. High voltage pulse application for the destruction of the Gram-negative bacterium Yersinia enterocolitica. Bioelectrochemistry and Bioenergetics. 43:135-141
Ma, L., Chang, F. J. and Barbosa-Cánovas, G. V. 1997. Inactivation of E. coli in liquid whole eggs using pulsed electric fields technologies. New frontiers in food engineering. Proceedings of the Fifth Conference of Food Engineering. American Institute of Chemical Engineers. 216-221.
Marquez, V. O., Mittal, G. S. and Griffiths, M. W. 1997. Destruction and inhibition of bacterial spores by high voltage pulsed electric field. J Food Sci. 62(2):399-401,409
Martin-Belloso, O., Vega-Mercado, H., Qin, B.-L., Chang, F.-J., Barbosa-Cánovas, G. V. and Swanson, B. G. 1997a. Inactivation of Escherichia coli suspended in liquid egg using pulsed electric fields. J Food Process Preserv. 21(3):193-208
Martin-Belloso, O., Qin, B. L., Chang, F. J., Barbosa-Cánovas, G. V. and Swanson, B. 1997b. Inactivation of Escherichia coli in skim milk by high intensity pulsed electric fields. J Food Process Eng. 20:317-336
Matsumoto, Y., Satake, T., Shioji, N. and Sakuma, A. 1991. Inactivation of microorganisms by pulsed high voltage applications. Conference Record of IEEE Industrial Applications Society Annual Meeting. 652-659.
Miller, J. F., Dower, W. J. and Tompkins, L. S. 1988. High-voltage electroporation of bacteria: Genetic transformation of Camylobacter jejuni with plasmid DNA. Proc Natl Acad Sci. 85:856-860
Mittal, G. S. and Choundry, M. 1997. Pulsed electric field sterilization of waste brine solution. Proceedings of the Seventh International Congress on Engineering and Food. Brighton Center, UK. C13-C16.
Mizuno, A. and Hori, Y. 1991. Destruction of living cells by pulsed high-voltage application. IEEE Trans Ind Appl. 24(3):387-394
Pagan, R., Esplugas, S., Gongora-Nieto, M. M., Barbosa-Cánovas, G. V. and Swanson, B. G. 1998. Inactivation of Bacillus subtilis spores using high intensity pulsed electric fields in combination with other food conservation technologies. Food Scie Technol Int. 4(1):33-44
Peleg, M. 1995. A model of microbial survival after exposure to pulse electric fields. J Sci Food Agric. 67(1):93-99
Pothakamury, U. R., Monsalve-Gonzalez, A., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995. Inactivation of Escherichia coli and Staphylococcus aureus in model foods by pulsed electric field technology. Food Res Int. 28(2):167-171
Pothakamury, U. R. 1995. High voltage pulsed electric field inactivation of Bacillus subtilis and Lactobacillus delbrueckii. Rev Esp C T. 35(1):101-107
Pothakamury, U. R., Vega, H., Zhang, Q. H., Barbosa-Cánovas, G. V. and Swanson, B. G. 1996. Effect of growth stage and processing temperature on the inactivation of E. coli by pulsed electric fields. J Food Protect. 59(11):1167-1171
Qin, B. L., Zhang, Q., Barbosa-Cánovas, G. V., Swanson, B. G. and Pedrow, P. D. 1994. Inactivation of microorganisms by pulsed electric fields with different voltage waveforms. IEEE Trans Dielec Insul. 1(6):1047-1057
Qin, B.-L., Chang, F.-J., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995a. Nonthermal inactivation of S. cerevisiae in apple juice using pulsed electric fields. Lebensm Wiss Technol. 28(6):564-568
Qin, B., Pothakamury, U. R., Vega, H., Martin, O., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995b. Food pasteurization using high intensity pulsed electric fields. J Food Technol. 49(12):55-60
Qin, B.-L., Zhang, Q. H., Barbosa-Cánovas, G. V., Swanson, B. G. and Pedrow, P. D. 1995c. Pulsed electric field treatment chamber design for liquid food pasteurization using a finite element method. Transactions of the ASAE. 38(2):557-565
Qin, B.-L., Barbosa-Cánovas, G. V., Swanson, B. G. and Pedrow, P. D. 1998. Inactivating microorganism using a pulsed electric field continuous treatment system. IEEE Trans Indus Applic. 34(1):43-49
Quass, D. W. 1997. Pulsed electric field processing in the food industry. A status report on PEF. Palo Alto, CA. Electric Power Research Institute. CR-109742.
Qui, X., Jia, M., Sharma, S., Tuhela, L. and Zhang, Q. H. 1998. An integrated PEF pilot plant for continuous nonthermal pasteurization of fresh orange juice. American Society of Agricultural Engineers. 41(4):1069-1074
Raso, J., Calderon, M. L., Gongora, M., Barbosa-Cánovas, G. V. and Swanson, B. G. 1998. Inactivation of Zygosaccharomyces Bailii in fruit juices by heat, high hydrostatic pressure and pulsed electric fields. J Food Sci. 63(6):1042-1044
Reina, L. D., Jin, Z. T., Yousef, A. E. and Zhang, Q. H. 1998. Inactivation of Listeria monocytogenes in milk by pulsed electric field. J Food Protect. 61(9):1203-1206
Sale, A. J. H. and Hamilton, W. A. 1967. Effects of high electric fields on microorganisms I. Killing of bacteria and yeast. Biochimt Biophys Acta. 148:781-788
Schoenbach, K. H., Peterkin, F. E., Alden, R. W. and Beebe, S. J. 1997. The effect of pulsed electric fields on biological cells: Experiments and applications. IEEE Trans Plasma Sci. 25(2):284-292
Sensoy, I., Zhang, Q. H. and Sastry, S. K. 1997. Inactivation kinetics of Salmonella dublin by pulsed electric field. J Food Process Eng. 20:367-381
Simpson, M. V., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995. The Combined inhibitory effect of lysozyme and high voltage pulsed electric fields on the growth of Bacillus subtilis spores. IFT Annual Meeting: Book of Abstracts. 267.
Sitzmann, V. 1995. High voltage pulse techniques for food preservation. G. W. Gould. New methods for food preservation. London, UK. Blackie Academic and Professional. 236-252.
Tsong, T. Y. 1990. Electrical modulation of membrane proteins: Enforced conformational oscillations and biological energy signals. Annu Rev Biophys Chem. 19:83-106
Vega-Mercado, H., Powers, J. R., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995. Plasmin inactivation with pulsed electric fields. J Food Sci. 60(5):1143-1146
Vega-Mercado, H., Martin-Belloso, O., Chang, F.-J., Barbosa-Cánovas, G. V. and Swanson, B. G. 1996a. Inactivation of Escherichia coli and Bacillus subtilis suspended in pea soup using pulsed electric fields. J Food Process Preserv. 20(6):501-510
Vega-Mercado, H., Pothakamury, U. R., Chang, F.-J., Barbosa-Cánovas, G. V. and Swanson, B. G. 1996b. Inactivation of Escherichia coli by combining pH, ionic strength and pulsed electric fields hurdles. Food Res Int. 29(2):117-121
Vega-Mercado, H. 1996c. Inactivation of proteolytic enzymes and selected microorganisms in foods using pulsed electric fields. Biological Systems Engineering. Pullman, WA. Washington State University.
Vega-Mercado, H., Martin-Belloso, O., Qin, B.-L., Chang, F.-J., Gongora-Nieto, M. M., Barbosa-Cánovas, G. V. and Swanson, B. G. 1997. Non-thermal food preservation: pulsed electric fields. Trends Food Sci Technol. 8(5):151-157
Vega-Mercado, H., Gongora-Nieto, M. M., Barbosa-Cánovas, G. V. and Swanson, B. G. 1999. Nonthermal preservation of liquid foods using pulsed electric fields. Handbook of Food Preservation. M. S. Rahman. Marcel Dekker, Inc. New York.
Yin, Y., Zhang, Q. H. and Sastry, S. K. 1997. High voltage pulsed electric field treatment chambers for the preservation of liquid food products. Ohio State University. US Patent 5,690,978.
Zhang, Q. H., Monsalve-Gonzalez, A., Barbosa-Cánovas, G. V. and Swanson, B. G. 1994a. Inactivation of E. coli and S. cerevisiae by pulsed electric fields under controlled temperature conditions. Transactions of the ASAE. 37(2):581-587
Zhang, Q. H., Chang, F.-J. and Barbosa-Cánovas, G. V. 1994b. Inactivation of microorganisms in a semisolid model food using high voltage pulsed electric fields. Lebensm Wiss Technol. 27(6):538-543
Zhang, Q. H., Qin, B.-L., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995a. Inactivation of E. coli for food pasteurization by high-strength pulsed electric fields. J Food Process Preserv. 19(2):103-118
Zhang, Q. H., Barbosa-Cánovas, G. V. and Swanson, B. G. 1995b. Engineering aspects of pulsed electric field pasteurization. J Food Eng. 25(2):261-281
Zhang, Q. H., Qiu, X. and Sharma, S. K. 1997. Recent development in pulsed electric field processing. Washington, DC. National Food Processors Association. New Technologies Yearbook. 31-42.
Zimmermann, U. and Benz, R. 1980. Dependence of the electrical breakdown voltage on the charging time in Valonia utricularis. J Membrane Biol. 53:33-43
Zimmermann, U. 1986. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 105:175-256
Click here to Receive our Amazing free newsletters
 |
"Royal rife developed "Five" Rife machine prototypes ,the fith rife machine(beam ray)was reported to be the most powerful" ""I can assure you that no one, not even myself, could help but be astounded at the results we are now obtaining with the assistance of our new machines and our new band of MOR's.(Mortal oscillatory rate)" (Letter from Dr. Johnson to Dr. Gruner (copy sent to Dr. Rife) dated, November 4, 1936."." The worlds most advanved multi purpose Fully Automatic Rife/Hoyland System Read more here
|